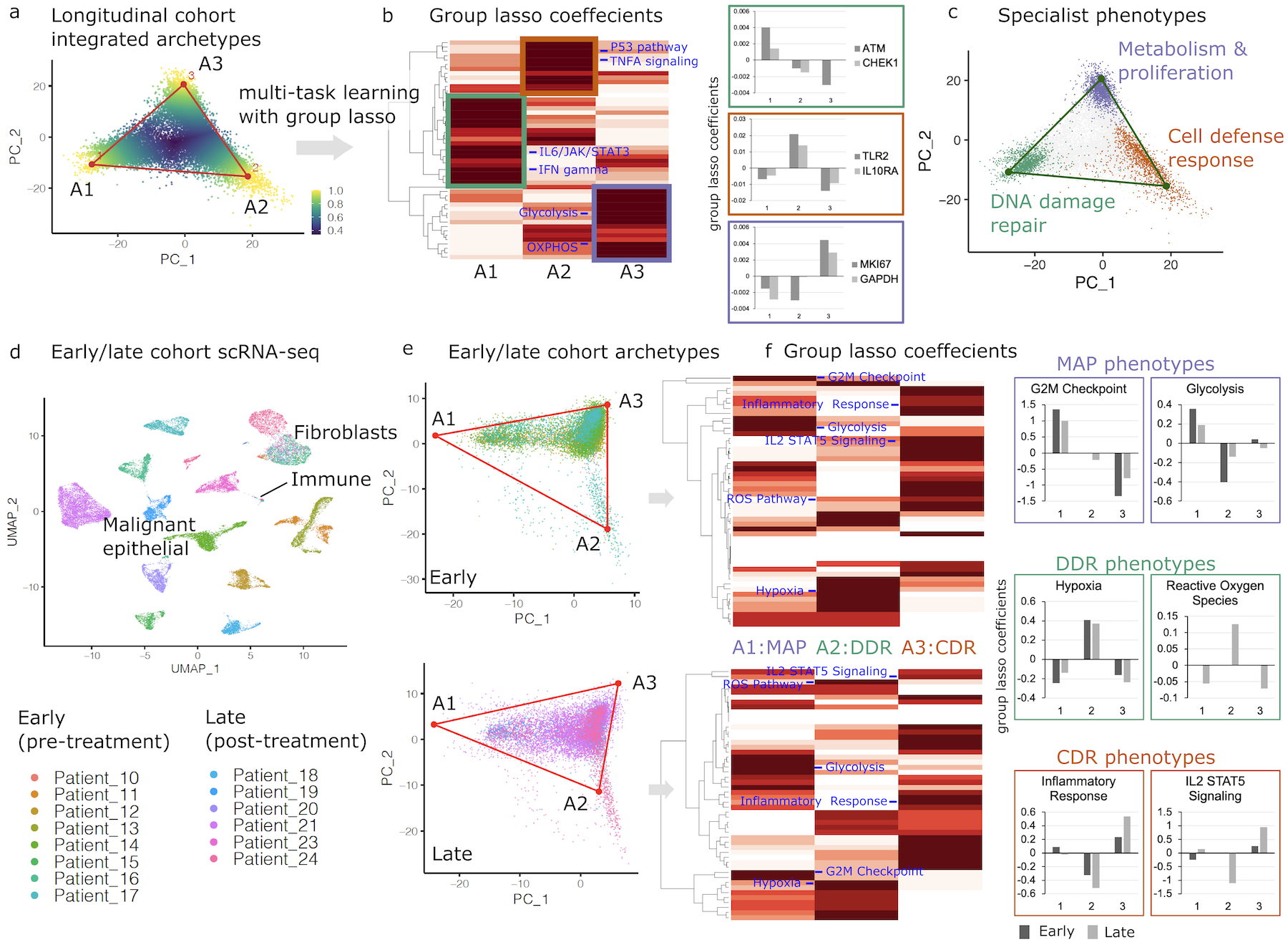
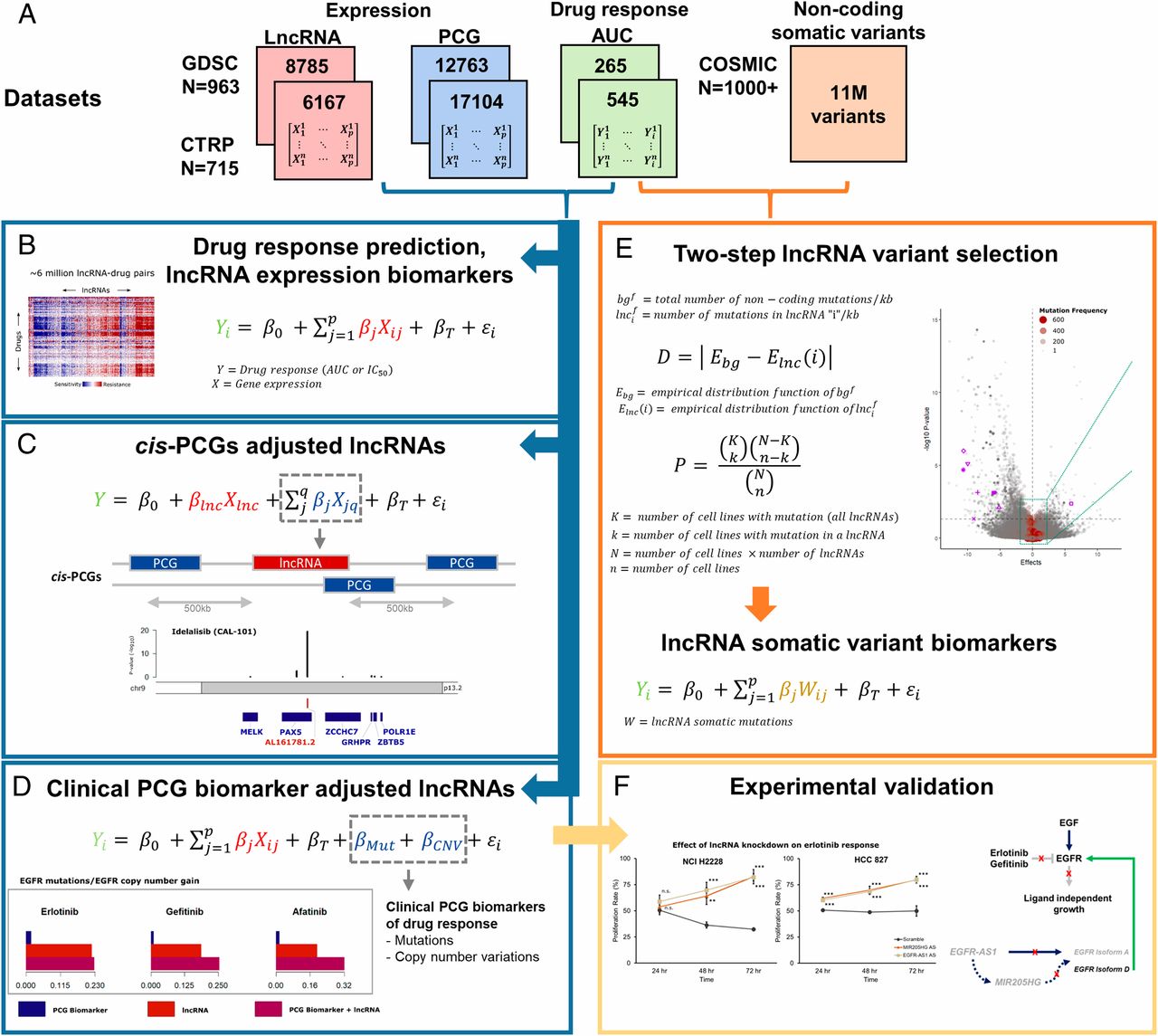
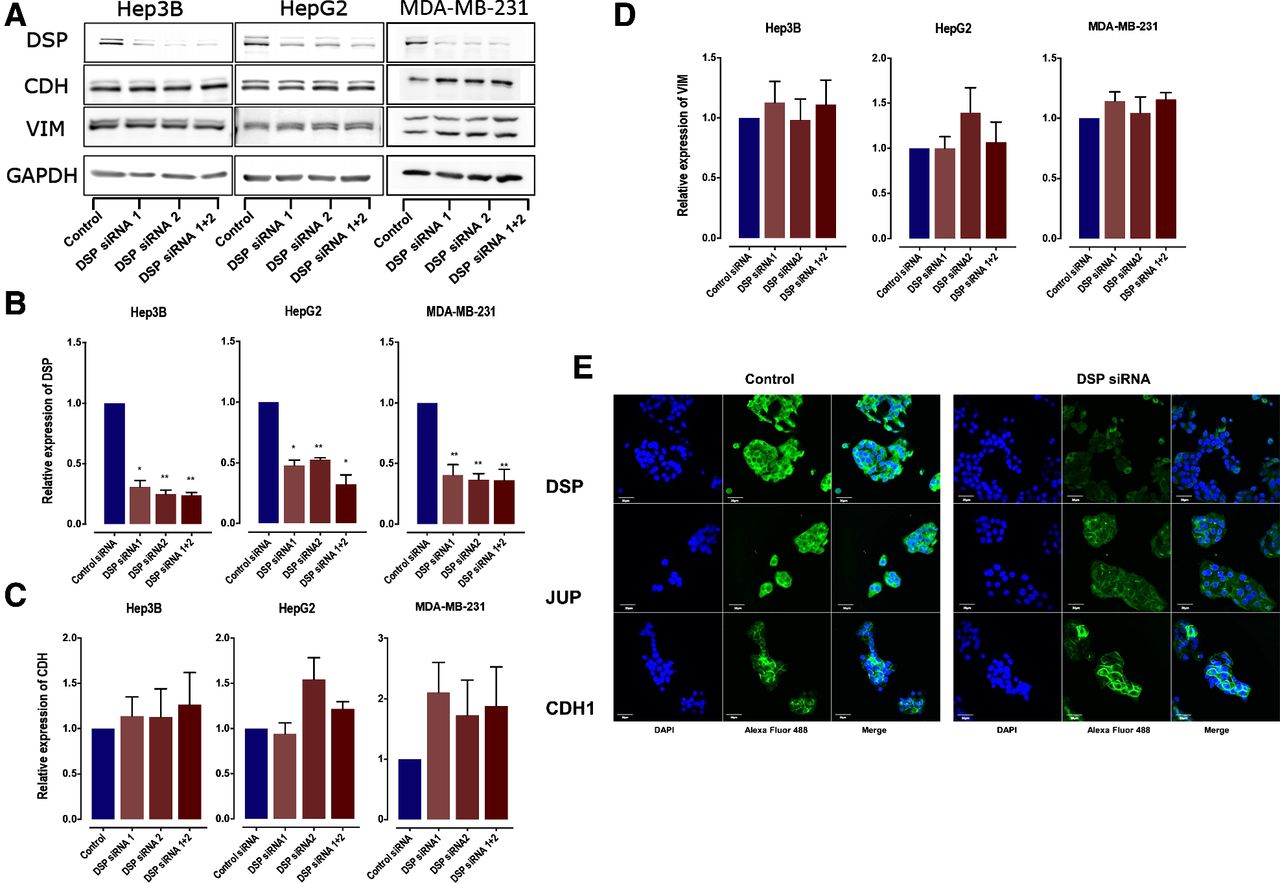
Single-cell genomics and cancer systems biology
Tumors are composed of a wide diversity of cancer and non-cancerous (normal, stromal, immune) cell populations. Even cancer cells within the same tumor exhibit a range of phenotypes owing to genetic and non-genetic heterogeneity. These properties have implications on both our understanding of how cancer cells originate and progress, and how they evolve over time, especially in response to treatment. My research aims at characterizing heterogeneity in human cancers and understand their impact on therapeutic response and patient outcomes. I use single-cell RNA-seq (scRNA-seq), bulk RNA-seq, whole exome/whole genome sequencing (WES/WGS) to study tumor evolution.
I am currently investigating the mechanisms of evolution of drug resistance in breast cancer, especially as they progress from primary to metastatic and refractory states. This work is supported by a pilot grant (PI: Nath) awarded via the US National Cancer Institute grant U54CA209978